【China Aluminum Network】 α-Al2O3 has excellent physical and chemical properties and is widely used in refractory, ceramic, chemical and other industrial fields. At present, α-Al2O3 is mainly produced by calcining industrial alumina in industrial production, but industrial alumina contains alkali metal oxide (exhibiting more prominently with Na2O), and high calcining temperature causes α-Al2O3 to have large grain size and agglomerates. Too many problems lead to a great influence on the high temperature performance of the prepared α-Al2O3. Therefore, a method of removing alkali metal oxides by sol-gel method, adding a mineralizer method, and a mechanical milling method to reduce the calcining temperature and controlling the grain morphology and the number of agglomerates has been developed.
The method of adding mineralizers mainly removes metal oxides in alkali industrial alumina by adding composite mineralizers such as H3BO3, TiO2, and MgCO3 to reduce the phase transition temperature and control the grain size and morphology. However, the addition of composite mineralizers may lead to overlapping effects and mutual reactions, which may affect the performance of α-Al2O3. The mechanical milling method has a high production cost, and the prepared α-Al2O3 has a low purity and is not suitable for industrial production. The sol-gel method is mainly to prepare aluminum oxide precursor by reacting aluminum salt with acid or alkali, and then calcining to prepare α-Al2O3. The α-Al2O3 obtained by this method has a low calcining temperature and a high purity, but the preparation process is complicated, and the purity of the raw material is required to be high, which cannot be implemented in large-scale industrial production. Therefore, how to improve on the basis of the sol-gel method, simplifying the preparation process while increasing the phase transformation rate and controlling the α-Al2O3 crystal morphology has become a problem that needs to be solved.
Studies have shown that the use of hydrochloric acid on the transition phase alumina or hydrated alumina to form a sol-gel, and then calcined at high temperatures to prepare α-Al2O3, can effectively reduce the operation process and reduce production costs. However, there are few researches on the effect of hydrochloric acid solution on the phase change of industrial alumina. For this reason, the researchers used industrial alumina as raw material to study the effect of hydrochloric acid solution with different pH values on the phase transformation during the calcination of industrial alumina.
Test method list
Test raw materials. The main raw materials are industrial alumina, analytically pure hydrochloric acid and α-Al2O3 fine powder (w≥99.99%). The chemical composition (w) of industrial alumina is: Al2O394.26%, Na2O0.51%, Fe2O30.12%, SiO20.07%, CaO0.02%, MgO0.01%, K2O0.025%, and the amount of burn reduction 4.98%; its main crystal phase is γ-Al2O3.
experiment method. The researchers used the following four steps to prepare α-Al2O3 powders:
According to the previous step, the ball mill was used to ball mill industrial alumina with a mass ratio of 4:1 balls and a ball milling time of 2 hours.
In the second step, concentrated analytical hydrochloric acid was diluted with deionized water into hydrochloric acid solutions with pH values of 1, 3, and 5, respectively.
In the third step, the measuring cylinders were respectively weighed with 300 mL of hydrochloric acid solution of different pH values, and 5 g of milled industrial alumina powder was added thereto, respectively, and stirred for 1 hour on a magnetic stirrer, and then the obtained emulsion was subjected to suction filtration treatment. A white precipitate (ie, alumina hydrate) was obtained and the precipitate was repeated three times as described above.
In the fourth step, the white precipitate (alumina hydrate) obtained at the end is dried at 110° C. for 12 h—24 h, and then the three acid-treated industrial aluminas and the non-acid treated industrial alumina are respectively °C, 900°C, 1000°C and 1100°C for 3h for calcination.
Performance characterization. The researchers used a laser particle size analyzer to measure the particle size of industrial alumina after ball milling. The X-ray diffractometer (Philips, X'Pert PRO, Cu Kα) was used to analyze the phase composition of the calcined sample, and α- was calculated according to the external standard method. The content of Al2O3 was calculated according to the Scherrer's formula, and the morphology and structure of the α-Al2O3 powder were observed by field emission scanning electron microscope (Nova 400 Nano).
Test results and discussion
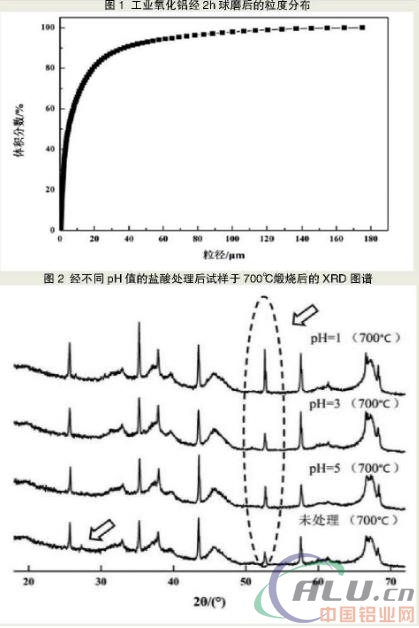
The particle size of the raw material after grinding. Figure 1 shows the particle size distribution of industrial alumina after 2 h ball milling. As can be seen from FIG. 1 , the d10 of industrial alumina after ball milling is set to be equal to 970 μm, d50=5.347 μm, and d90=33.224 μm. The ball milling process effectively reduces the particle size of the industrial alumina, thereby increasing the phase transformation conversion rate of the alumina. This is because the smaller the particle size, the larger the specific surface area of the reaction system, the corresponding increase of the reaction interface and diffusion cross section, the flattening of the bond strength distribution curve, and the increase in the proportion of weak bonds, so the reaction and diffusion capacity are improved.
The phase and grain size of the sample after calcination. The XRD patterns of different samples calcined at 700°C-1100°C show that as the calcination temperature increases, the diffraction peaks of α-Al2O3 in each sample gradually increase, and the peaks of the transition phases γ-Al2O3 and θ-Al2O3. Gradually weakened, θ-Al2O3 almost completely disappeared at a calcining temperature of 1100°C. This is due to the fact that as the temperature rises, the activity of the transition phase Al2O3 gradually increases, and the driving force of the phase transformation is increased, and the transition phase Al2O3 continuously changes to the stable phase α-Al2O3.
The XRD pattern of the powder obtained by calcining different samples at 700°C is shown in FIG. 2 . Figure 2 shows that after calcination at 700 °C, the α-Al2O3 diffraction peak in the samples after the acid treatment is stronger than that without the acid treatment, and there are also some unique transitional phases of Al2O3 diffraction in the samples without acid treatment. peak. This shows that after the acid treatment, the α-transformation rate of the sample is higher than that of the untreated one. After analysis, the phase transition path is as follows: a variety of amorphous alumina hydrate → amorphous alumina → γ-Al2O3 → θ-Al2O3 + α-Al2O3 → α-Al2O3. It can be seen that the alumina in the sample can undergo transition between transition phases and transition to a stable phase between α phases at a low temperature, thereby lowering the phase transition temperature and increasing the α transformation transformation rate.
The content of α-Al2O3 calculated by external standard method is shown in Table 1. From Table 1, it can be seen that the α-transformation conversion rate of alumina is higher than that of the non-hydrochloric acid-treated sample at different calcining temperatures. This may be because the amorphous alumina precursor formed after the acid treatment can accelerate the transition of the transition phase alumina to the α phase at a lower temperature, and the α-Al2O3 crystal grains formed as a seed crystal accelerates α again. Phase change. Comparing the α-phase conversion rate of the samples treated with different pH value of hydrochloric acid solution, it can be seen that the content of α-Al2O3 in the samples after calcination gradually increases with the decrease of the pH of the hydrochloric acid solution. This may be due to the different types of amorphous alumina precursors produced after treatment of industrial alumina with different pH values, resulting in different phase transition rates.
The researchers calculated the grain size of α-Al2O3 in samples treated with hydrochloric acid of different pH values and calcined at different temperatures. The results are shown in Table 2. From Table 2, it can be seen that as the calcination temperature and the concentration of the hydrochloric acid solution increase, the grain size of α-Al 2 O 3 in the sample gradually increases.
Microstructure analysis. The microstructures of the samples treated with hydrochloric acid solutions of different pH values after calcination at 700° C., 1000° C., and 1100° C. showed that when the calcination temperature was the same, as the concentration of the hydrochloric acid solution decreased, the alumina crystal particles were agglomerated and crystals were crystallized. The particle size is too small, which is consistent with the results calculated by the Scherrer formula.
When the calcining temperature is 700°C, due to the low calcination temperature, the transition between the transition phases is mainly in the sample, and only a very small amount of the aluminum oxide undergoes α phase transition. The microstructure of the sample exhibits a transitional phase and Amorphous Alumina Agglomeration.
When the calcination temperature is higher than 1000°C, there is a pronounced worm-like and layered structure in the sample after acid treatment compared to the sample without hydrochloric acid treatment, and the smaller the pH value of the hydrochloric acid solution, the more worms in the sample. The more obvious the shape and layered structure. Some scholars have found that when industrial alumina is used as a raw material and calcined at 1400°C, α-Al2O3 obtained without adding a mineralizer is similar to a worm-like spatial network structure; when mineralizing agent AlF3 is added, it is obtained. α-Al2O3 is a platy crystal form. It can be inferred that, after treatment with hydrochloric acid, the formation of a part of α-Al2O3 from solid-phase mass transfer to gas-phase mass transfer at high temperature can be changed, and the crystallization process and crystallization habit of alumina crystals can be changed to form a layered structure.
With the increase of calcination temperature, the alumina particles in the sample continue to agglomerate. When the calcination temperature is 1100°C, large α-Al2O3 grains begin to appear in the sample. With the increase of the pH value of the hydrochloric acid solution, particle agglomeration becomes more obvious. . This may be due to the effect of different α-Al2O3 seed crystal content on the morphology of the alumina grains after treatment with different pH values of hydrochloric acid solution.
In summary, the acid oxides of industrial alumina were treated with hydrochloric acid solutions of different pH values, and then α-Al2O3 was calcined at 700°C-1100°C. As the pH of the hydrochloric acid solution decreased, the samples were oxidized after calcination. The transformation rate of α-transformation of aluminum gradually increases, and the grain size of α-Al2O3 gradually increases. When the hydrochloric acid solution pH=1, the sample was calcined at 1100°C to complete the alpha phase transformation of alumina. The grain size of α-Al2O3 was 91 nm, and it was generated by sheet and worm-like structure. The number of agglomerates in the sample was also obtained. less. It can be seen that the hydrochloric acid treatment plays an important role in promoting the alumina alpha phase transformation during the calcination of industrial alumina, changing the grain size, lowering the agglomerate particles, and improving the high temperature performance of the product.